Exploiting nonspecific effects and innate immune memory for greater vaccine protection
Samuel Pasco
Early Stage Researcher 7, CIC bioGUNE | Center for Cooperative Research in Biosciences
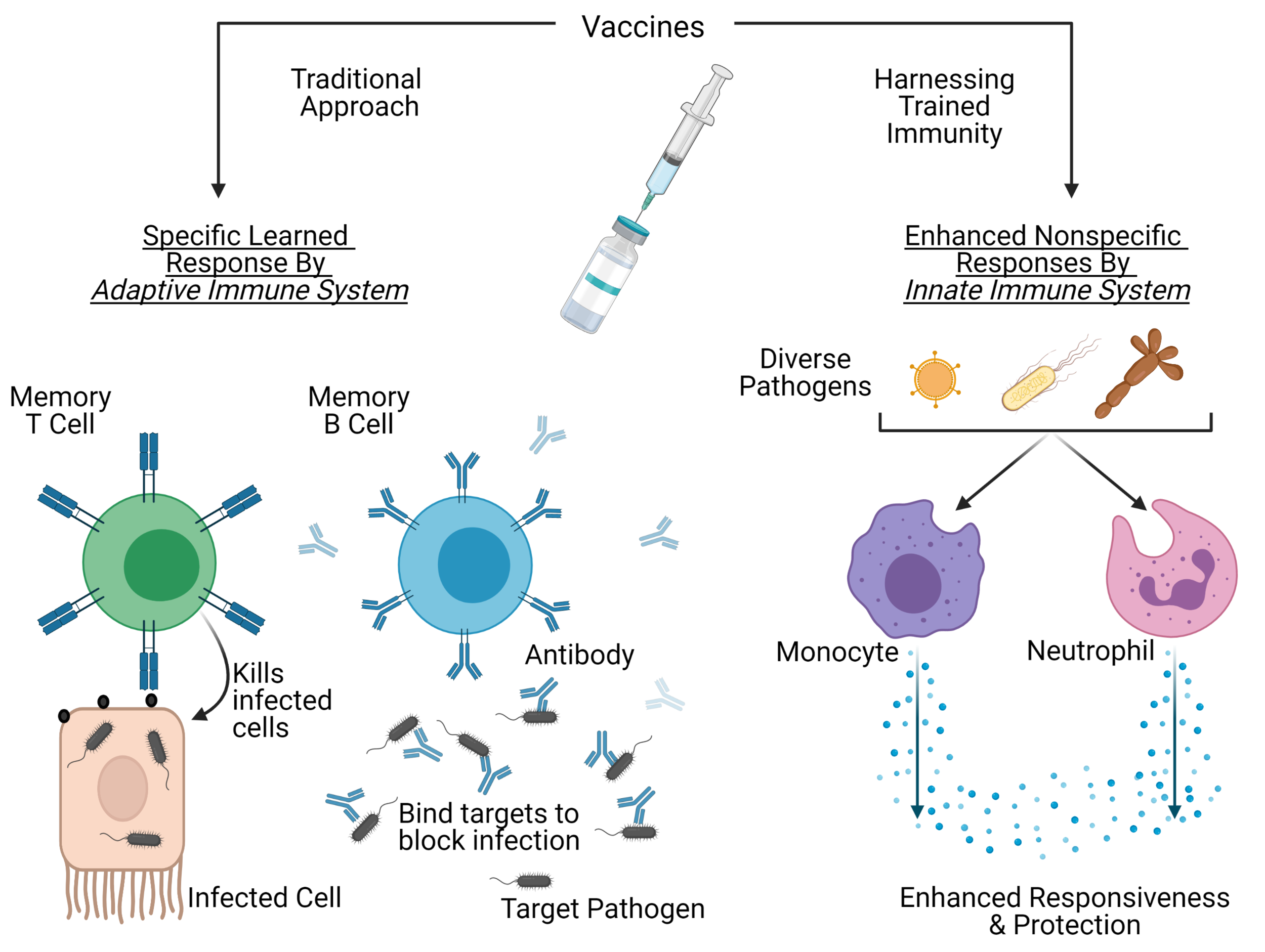
Vaccine protection can be increased through nonspecific responses of the innate immune system. Figure created with BioRender.com.
Vaccines continue to be medicine’s greatest preventative intervention. They work by inducing and preserving a protective immune response to a target pathogen to prevent future infection. By administering a formulation of microbial components or weakened pathogens, vaccines aim to create a specific "learned" response generated by the adaptive immune system. In addition to protection against the target diseases, some vaccines also appear to confer some general protection against other non-related infections as well.
Increasing epidemiological data have revealed that administration of live-attenuated vaccines (such as Bacillus Calmette-Guérin (BCG), measles, and oral polio vaccines), correlates with overall increased survival, particularly with babies (1). These mechanisms do not associate with specific vaccine responses from the adaptive immune system, but rather point to enhanced nonspecific responses by innate immune cells (2).
The innate immune system is the body’s first immunological line of defence, consisting of cells that respond immediately and non-specifically to foreign microbes. White blood cells, such as monocytes and neutrophils, circulate in the blood and aggressively fight invading microbes, thus preventing infection. These cells have historically been ignored in vaccine development because they do not develop pathogen-specific memory.
Unlike specific responses generated by the adaptive immune system, the nonspecific effects induced by these vaccines are caused by a phenomenon commonly called “trained immunity”. Trained immunity refers to long-term functional changes in innate cells, that are epigenetically and metabolically modified after primary exposure. This leads to increased secondary responses to stimulation by target and non-target pathogens (3). The resulting cells produce more pathogen receptors and inflammatory signalling molecules that enhance the response to infection.
The most extensively studied vaccine that induces trained immunity is BCG, which was originally developed almost a hundred years ago and is administered to millions of newborns each year. The presence of a BCG scar on children is associated with a 39% decrease in mortality (4). For months after vaccination, innate cells in both infants (5) and adults (6) become more responsive to pathogens and their molecules. Though the exact mechanisms are not fully understood, it has been shown that BCG vaccination in adults causes changes in bone marrow hematopoietic stem cells, which produce innate immune cells (7). These changes result in the production of monocytes and neutrophils (8) that respond more robustly to stimulation with pathogens and inflammatory molecules.
To examine how these enhancements affect infection, volunteers received the BCG vaccination prior to experimental infection with the yellow fever virus. BCG recipients were shown to have better control of the infection, associated with increased signalling responses (9). Additional protective effects were demonstrated in a double-blind, randomised clinical trial, where BCG offered protection to elderly patients by reducing incidences of infection, particularly against viral respiratory tract infections (10). These off-target protective effects could be harnessed for other vaccine formulations, as many antimicrobial immune responses rely on non-adaptive mechanisms to prevent or clear infection. Additionally, because innate defences are the first component that pathogens encounter, enhancing these cellular components could provide additional protection (11).
This strategy could prove successful, particularly in the fight against the rise of antimicrobial resistance (AMR). Vaccines against AMR bacteria have several potential benefits:
(i) unlike antibiotics, vaccines generally do not induce resistance, especially when given prophylactically.
(ii) new technologies support vaccine development and outpace the discovery of new antibiotics.
(iii) vaccination can reduce the incidence of resistant bacterial disease and antibiotic use.
(iv) vaccine administration will not disrupt the microbiome of the recipient (12).
Broad spectrum, nonspecific immunisations mediated by innate mechanisms could revolutionize vaccine design. By focusing on how future vaccines would confer protection like BCG, researchers will unlock the untapped potential of innate immune cells.
References:
De Bree, et al. Semin Immunol. 2018 Oct; 39:35-43. https://doi.org/10.1016/j.smim.2018.06.002
Uthayakumar, et al. Front Immunol. 2018 Dec 4; 9:2869. https://doi.org/10.3389/fimmu.2018.02869
Netea, et al. Nat Rev Immunol . 2020 Jun;20(6):375-388. https://doi.org/10.1038/s41577-020-0285-6
Benn, et al. J Intern Med . 2020 Dec;288(6): 614-624. https://doi.org/10.1111/joim.13084
Jensen, et al. J Infect Dis. 2015 Mar 15;211(6): 956-67 https://doi.org/10.1093/infdis/jiu508
Kleinnijenhuis, et al. Proc Natl Acad Sci USA. 2012 Oct 23; 109(43):17537-42 https://doi.org/10.1073/pnas.1202870109
Cirovic, et al. Cell Host Microbe. 2020 Aug 12; 28(2):322-334.e5 https://doi.org/10.1016/j.chom.2020.05.014
Moorlag, et al. Cell Rep. 2020 Nov 17; 33(7):108387 https://doi.org/10.1016/j.celrep.2020.108387
Arts, et al. Cell Host Microbe. 2018 Jan 10; 23(1):89-100.e5 https://doi.org/10.1016/j.chom.2017.12.010
Giamarellos-Bourboulis, et al. Cell. 2020 Oct 15; 183(2):315-323.e9 https://doi.org/10.1016/j.cell.2020.08.051
Pasco and Anguita. Cells. 2020 Sep 16; 9(9):2109 https://doi.org/10.3390/cells9092109
Bloom, et al. Proc Natl Acad Sci USA. 2018 Dec 18; 115(51):12868-12871 https://doi.org/10.1073/pnas.1717157115
-
RT @mcclean_siobhan: Our fantastic @Bactivax meeting is all over. Great science, great chats and plenty of fun. Thanks to Rita Berisio (… https://t.co/sC7ddNvinE
-
RT @mcclean_siobhan: We had a really informative session this morning on D3 of our @BactiVax summer school: Science Communication & over… https://t.co/sj3zYNHs0h
-
RT @mcclean_siobhan: Amazing talk from Mariagrazia Pizza from @GSK who shared her extensive experience in working on Bacterial #Vaccines… https://t.co/Nm4U46hxJd
-
RT @mcclean_siobhan: Amazing day and it’s been wonderful to see the research projects of each #ESRs develop and grow. 🎉🎉🎉 @REA_research
-
RT @mcclean_siobhan: @LorenzoBossi5 was our last speaker of the day. Based at @immunxperts, he presented his work on the human immune re… https://t.co/THA1OkaxT3