2023
New Glucosamine-Based TLR4 Agonists: Design, Synthesis, Mechanism of Action, and In Vivo Activity as Vaccine Adjuvants
Alessio Romerio, Nicole Gotri, Ana Rita Franco, Valentina Artusa, Mohammed Monsoor Shaik, Samuel T. Pasco, Unai Atxabal, Alejandra Matamoros-Recio, Marina Mínguez-Toral, Juan Diego Zalamea, Antonio Franconetti, Nicola G. A. Abrescia, Jesus Jimenez-Barbero, Juan Anguita, Sonsoles Martín-Santamaría, and Francesco Peri*
We disclose here a panel of small-molecule TLR4 agonists (the FP20 series) whose structure is derived from previously developed TLR4 ligands (FP18 series). The new molecules have increased chemical stability and a shorter, more efficient, and scalable synthesis. The FP20 series showed selective activity as TLR4 agonists with a potency similar to FP18. Interestingly, despite the chemical similarity with the FP18 series, FP20 showed a different mechanism of action and immunofluorescence microscopy showed no NF-κB nor p-IRF-3 nuclear translocation but rather MAPK and NLRP3-dependent inflammasome activation. The computational studies related a 3D shape of FP20 series with agonist binding properties inside the MD-2 pocket. FP20 displayed a CMC value lower than 5 μM in water, and small unilamellar vesicle (SUV) formation was observed in the biological activity concentration range. FP20 showed no toxicity in mouse vaccination experiments with OVA antigen and induced IgG production, thus indicating a promising adjuvant activity. http://dx.doi.org/10.1021/acs.jmedchem.2c01998
Download full-text PDF here
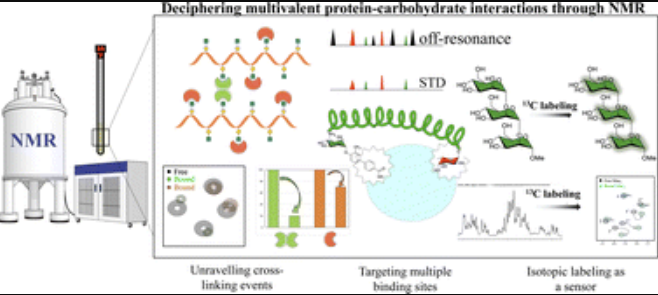
Exploring multivalent carbohydrate–protein interactions by NMR
Jon I. Quintana, Unai Atxabal, Luca Unione, Ana Ardá and Jesús Jiménez-Barbero
Nuclear Magnetic Resonance (NMR) has been widely employed to assess diverse features of glycan–protein molecular recognition events. Different types of qualitative and quantitative information at different degrees of resolution and complexity can be extracted from the proper application of the available NMR-techniques. In fact, affinity, structural, kinetic, conformational, and dynamic characteristics of the binding process are available. Nevertheless, except in particular cases, the affinity of lectin-sugar interactions is weak, mostly at the low mM range. This feature is overcome in biological processes by using multivalency, thus augmenting the strength of the binding. However, the application of NMR methods to monitor multivalent lectin–glycan interactions is intrinsically challenging. It is well known that when large macromolecular complexes are formed, the NMR signals disappear from the NMR spectrum, due to the existence of fast transverse relaxation, related to the large size and exchange features. Indeed, at the heart of the molecular recognition event, the associated free-bound chemical exchange process for both partners takes place in a particular timescale. Thus, these factors have to be considered and overcome. In this review article, we have distinguished, in a subjective manner, the existence of multivalent presentations in the glycan or in the lectin. From the glycan perspective, we have also considered whether multiple epitopes of a given ligand are presented in the same linear chain of a saccharide (i.e., poly-LacNAc oligosaccharides) or decorating different arms of a multiantennae scaffold, either natural (as in multiantennae N-glycans) or synthetic (of dendrimer or polymer nature). From the lectin perspective, the presence of an individual binding site at every monomer of a multimeric lectin may also have key consequences for the binding event at different levels of complexity. http://dx.doi.org/10.1039/d2cs00983h
Download full-text PDF here
2022
Structures of the Inhibitory Receptor Siglec-8 in Complex with a High-Affinity Sialoside Analogue and a Therapeutic Antibody
Maria Pia Lenza, Unai Atxabal, Corwin Nycholat, Iker Oyenarte, Antonio Franconetti, Jon Imanol Quintana, Sandra Delgado, Reyes Núñez-Franco, Carmen Teresa Garnica Marroquín, Helena Coelho, Luca Unione, Gonzalo Jiménez-Oses, Filipa Marcelo, Mario Schubert, James C. Paulson, Jesús Jiménez-Barbero*, and June Ereño-Orbea
Human sialic acid binding immunoglobulin-like lectin-8 (Siglec-8) is an inhibitory receptor that triggers eosinophil apoptosis and can inhibit mast cell degranulation when engaged by specific monoclonal antibodies (mAbs) or sialylated ligands. Thus, Siglec-8 has emerged as a critical negative regulator of inflammatory responses in diverse diseases, such as allergic airway inflammation. Herein, we have deciphered the molecular recognition features of the interaction of Siglec-8 with the mAb lirentelimab (2C4, under clinical development) and with a sialoside mimetic with the potential to suppress mast cell degranulation. The three-dimensional structure of Siglec-8 and the fragment antigen binding (Fab) portion of the anti-Siglec-8 mAb 2C4, solved by X-ray crystallography, reveal that 2C4 binds close to the carbohydrate recognition domain (V-type Ig domain) on Siglec-8. We have also deduced the binding mode of a high-affinity analogue of its sialic acid ligand (9-N-napthylsufonimide-Neu5Ac, NSANeuAc) using a combination of NMR spectroscopy and X-ray crystallography. Our results show that the sialoside ring of NSANeuAc binds to the canonical sialyl binding pocket of the Siglec receptor family and that the high affinity arises from the accommodation of the NSA aromatic group in a nearby hydrophobic patch formed by the N-terminal tail and the unique G–G′ loop. The results reveal the basis for the observed high affinity of this ligand and provide clues for the rational design of the next generation of Siglec-8 inhibitors. Additionally, the specific interactions between Siglec-8 and the N-linked glycans present on the high-affinity receptor FcεRIα have also been explored by NMR. https://doi.org/10.1021/jacsau.2c00592
Download full-text PDF here
Molecular Immunology in Bacterial Vaccine Discovery
Rita Berisio (Editorial)
The global threat of antimicrobial resistance (AMR) poses a difficult challenge, as underscored by the World Health Organization (WHO), which identifies AMR as one of the three greatest threats to human health. Annual deaths due to AMR-related infections are ~700,000 and are projected to rise up to 10 million by 2050. New antibiotics are not a solution since bacteria promptly adapt and develop new resistance mechanisms. Therefore, there is a strong need to invest in vaccines against AMR infections.
This Special Issue offers an open access forum that aims to bring together a collection of nine review articles addressing multi-disciplinary approaches to unveil various aspects of vaccine development against human bacterial pathogens. Specifically, it focuses on a panel of harmful pathogens, including Mycobacterium tuberculosis (Mtb), AMR Burkholderia species, and selected ESKAPE pathogens. Among ESKAPE pathogens, a group of multidrug-resistant bacteria that are the leading cause of hospital infections globally and “escape” the biocidal action of antibiotics, we focus on Pseudomonas aeruginosa and Enterococci, both leading causes of death worldwide. https://doi.org/10.3390/cells11233803
Download full-text PDF here
HLA-DR polymorphism in SARS-CoV-2 infection and susceptibility to symptomatic COVID-19
Stuart Astbury, Catherine J. Reynolds, David K. Butler, Diana C. Muñoz-Sandoval, Kai-Min Lin, Franziska P. Pieper, Ashley Otter, Afroditi Kouraki, Lola Cusin, Jessica Nightingale, Amrita Vijay, Simon Craxford, Guruprasad P. Aithal, Patrick J. Tighe, Joseph M. Gibbons, Corinna Pade, George Joy, Mala Maini, Benny Chain, Amanda Semper, Timothy Brooks, Benjamin J. Ollivere, Áine McKnight, Mahdad Noursadeghi, Thomas A. Treibel, Charlotte Manisty, James C. Moon, COVIDsortium Investigators*, Ana M. Valdes, Rosemary J. Boyton, Daniel M. Altmann 2022 https://doi.org/10.1111/imm.13450
SARS-CoV-2 infection results in different outcomes ranging from asymptomatic infection to mild or severe disease and death. Reasons for this diversity of outcome include differences in challenge dose, age, gender, comorbidity and host genomic variation. Human leukocyte antigen (HLA) polymorphisms may influence immune response and disease outcome. We investigated the association of HLAII alleles with case definition symptomatic COVID-19, virus-specific antibody and T-cell immunity. A total of 1364 UK healthcare workers (HCWs) were recruited during the first UK SARS-CoV-2 wave and analysed longitudinally, encompassing regular PCR screening for infection, symptom reporting, imputation of HLAII genotype and analysis for antibody and T-cell responses to nucleoprotein (N) and spike (S). Of 272 (20%) HCW who seroconverted, the presence of HLA-DRB1*13:02 was associated with a 6·7-fold increased risk of case definition symptomatic COVID-19. In terms of immune responsiveness, HLA-DRB1*15:02 was associated with lower nucleocapsid T-cell responses. There was no association between DRB1 alleles and anti-spike antibody titres after two COVID vaccine doses. However, HLA DRB1*15:01 was associated with increased spike T-cell responses following both first and second dose vaccination. Trial registration: NCT04318314 and ISRCTN15677965
Download full-text PDF here
2021
Schematic approach for using bioinformatic tools to predict immunogenic epitopes and their antigenicity, allergenicity and toxicity.
Predictive and Experimental Immunogenicity of Burkholderia Collagen-like Protein 8-Derived Antigens Megan E. Grund, Eliza Kramarska, Soo Jeon Choi, Dudley H. McNitt, Christopher P. Klimko, Nathaniel O. Rill, Jennifer L. Dankmeyer, Jennifer L. Shoe, Melissa Hunter, David P. Fetterer, Zander M. Hedrick, Ivan Velez, Sergei S. Biryukov, Christopher K. Cote, Rita Berisio, and Slawomir Lukomski. Vaccines 2021, 9(11), 1219; https://doi.org/10.3390/vaccines9111219.
Burkholderia pseudomallei is an infectious bacterium of clinical and biodefense concern, and is the causative agent of melioidosis. The mortality rate can reach up to 50% and affects 165,000 people per year; however, there is currently no vaccine available. In this study, we examine the antigen-specific immune response to a vaccine formulated with antigens derived from an outer membrane protein in B. pseudomallei, Bucl8. Here, we employed a number of bioinformatic tools to predict Bucl8-derived epitopes that are non-allergenic and non-toxic, but would elicit an immune response. From these data, we formulated a vaccine based on two extracellular components of Bucl8, the β-barrel loops and extended collagen and non-collagen domains. Outbred CD-1 mice were immunized with vaccine formulations—composed of recombinant proteins or conjugated synthetic peptides with adjuvant—to assess the antigen-specific immune responses in mouse sera and lymphoid organs. We found that mice vaccinated with either Bucl8-derived components generated a robust TH2-skewed antibody response when antigen was combined with the adjuvant AddaVax, while the TH1 response was limited. Mice immunized with synthetic loop peptides had a stronger, more consistent antibody response than recombinant protein antigens, based on higher IgG titers and recognition of bacteria. We then compared peptide-based vaccines in an established C57BL/6 inbred mouse model and observed a similar TH2-skewed response. The resulting formulations will be applied in future studies examining the protection of Bucl8-derived vaccines.
Download full-text PDF here.
Arginine-rich C-terminal domain specifically binds cardiolipin and phosphorylated phosphatidylinositols.
Flavio De Maio, Alessandro Salustri, Basem Battah, Ivana Palucci, Federica Marchionni, Silvia Bellesi, Valentina Palmieri, Massimiliano Papi, Eliza Kramarska, Maurizio Sanguinetti, Michela Sali, Rita Berisio, and Giovanni Delogu. Virulence 2021, 12:1, 868-884, DOI: 10.1080/21505594.2021.1897247.
PE_PGRS proteins of Mycobacterium tuberculosis (Mtb) constitute a large family of complex modular proteins whose role is still unclear. Among those, we have previously shown, using the heterologous expression in Mycobacterium smegmatis, that PE_PGRS3 containing a unique arginine-rich C-terminal domain, promotes adhesion to host cells. In this study, we investigate the role of PE_PGRS3 and its C-terminal domain directly in Mtb using functional deletion mutants. The results obtained here show that PE_PGRS3 is localized on the mycobacterial cell wall and its arginine-rich C-terminal region protrudes from the mycobacterial membrane and mediates Mtb entry into epithelial cells. Most importantly, this positively charged helical domain specifically binds phosphorylated phosphatidylinositols and cardiolipin, whereas it is unable to bind other phospholipids. Interestingly, administration of cardiolipin and phosphatidylinositol but no other phospholipids was able to turn-off expression of pe_pgrs3 activated by phosphate starvation conditions. These findings suggest that PE_PGRS3 has the key role to serve as a bridge between mycobacteria and host cells by interacting with specific host phospholipids and extracting them from host cells, for their direct integration or as a source of phosphate, during phases of TB pathogenesis when Mtb is short of phosphate supply.
Download full-text PDF here.
The main virulence factors of Pseudomonas aeruginosa and the adaptations it undergoes to persist in hostile environments such as the respiratory tract of patients with cystic fibrosis (CF).
Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors Irene Jurado-Martín, Maite Sainz-Mejías and Siobhán McClean. Int. J. Mol. Sci. 2021, 22(6), 3128; https://doi.org/10.3390/ijms22063128.
Pseudomonas aeruginosa is a dominant pathogen in people with cystic fibrosis (CF) contributing to morbidity and mortality. Its tremendous ability to adapt greatly facilitates its capacity to cause chronic infections. The adaptability and flexibility of the pathogen are afforded by the extensive number of virulence factors it has at its disposal, providing P. aeruginosa with the facility to tailor its response against the different stressors in the environment. A deep understanding of these virulence mechanisms is crucial for the design of therapeutic strategies and vaccines against this multi-resistant pathogen. Therefore, this review describes the main virulence factors of P. aeruginosa and the adaptations it undergoes to persist in hostile environments such as the CF respiratory tract.
Download full-text PDF here.
Overview of mechanisms of pathogenesis and virulence traits modulated by neuropeptides. The question mark indicates that the given mechanism is putative.
Mammalian Neuropeptides as Modulators of Microbial Infections: Their Dual Role in Defense versus Virulence and Pathogenesis Daria Augustyniak, Eliza Kramarska, Paweł Mackiewicz, Magdalena Orczyk-Pawiłowicz and Fionnuala T. Lundy. Int. J. Mol. Sci. 2021, 22(7), 3658; https://doi.org/10.3390/ijms22073658.
The regulation of infection and inflammation by a variety of host peptides may represent an evolutionary failsafe in terms of functional degeneracy and it emphasizes the significance of host defense in survival. Neuropeptides have been demonstrated to have similar antimicrobial activities to conventional antimicrobial peptides with broad-spectrum action against a variety of microorganisms. Neuropeptides display indirect anti-infective capacity via enhancement of the host’s innate and adaptive immune defense mechanisms. However, more recently concerns have been raised that some neuropeptides may have the potential to augment microbial virulence. In this review we discuss the dual role of neuropeptides, perceived as a double-edged sword, with antimicrobial activity against bacteria, fungi, and protozoa but also capable of enhancing virulence and pathogenicity. We review the different ways by which neuropeptides modulate crucial stages of microbial pathogenesis such as adhesion, biofilm formation, invasion, intracellular lifestyle, dissemination, etc., including their anti-infective properties but also detrimental effects. Finally, we provide an overview of the efficacy and therapeutic potential of neuropeptides in murine models of infectious diseases and outline the intrinsic host factors as well as factors related to pathogen adaptation that may influence efficacy.
Download full-text PDF here.
Domain organization and sequence of PE_PGRS33. In the PE domain, the conserved PE motif is colored red. In the PGRS domain, sequences of the four PGII sandwich motifs are colored green, purple, blue, and cyan.
PE_PGRS33, an Important Virulence Factor of Mycobacterium tuberculosis and Potential Target of Host Humoral Immune Response Eliza Kramarska, Flavia Squeglia, Flavio De Maio, Giovanni Delogu and Rita Berisio. Cells 2021, 10(1), 161; https://doi.org/10.3390/cells10010161.
PE_PGRS proteins are surface antigens of Mycobacterium tuberculosis (Mtb) and a few other pathogenic mycobacteria. The PE_PGRS33 protein is among the most studied PE_PGRSs. It is known that the PE domain of PE_PGRS33 is required for the protein translocation through the mycobacterial cell wall, where the PGRS domain remains available for interaction with host receptors. Interaction with Toll like receptor 2 (TLR2) promotes secretion of inflammatory chemokines and cytokines, which are key in the immunopathogenesis of tuberculosis (TB). In this review, we briefly address some key challenges in the development of a TB vaccine and attempt to provide a rationale for the development of new vaccines aimed at fostering a humoral response against Mtb. Using PE_PGRS33 as a model for a surface-exposed antigen, we exploit the availability of current structural data using homology modeling to gather insights on the PGRS domain features.
Download full-text PDF here.
Overview of Proposed Mechanisms of Action of Adjuvants in vaccines. (A) Receptors and Molecule Activation of adjuvants. The active principle of GLA-SE and AS0, the MPLA, activates TLR4 and downstream pathways, namely MyD88 and, after internalization of the CD14/TLR4/MD-2 complex, the “late” TRAM-TRIF pathway. Starch interacts with C-type DC-SIGN receptor. IC31, more specifically, CpG motifs, as well as pDNA present in TMC microparticles activate TLR9 inside the endosome. AS01 and Chitosan activate the inflammasome and promote the subsequent release of pro-inflammatory cytokines. CDNs activate STING receptors in the endoplasmic reticulum. (B) IC31′s KLK peptide increases the permeability of the adjuvant system in the membrane to deliver the antigen and CpG motifs. (C) Cationic Liposome adjuvants, such as CAF01, promote a depot effect on the injection site and release the antigen in a controlled way, stimulating innate immunity.
Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants Ana Rita Franco and Francesco Peri. Cells 2021, 10(1), 78; https://doi.org/10.3390/cells10010078.
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis (Mtb) that sits in the top 10 leading causes of death in the world today and is the current leading cause of death among infectious diseases. Although there is a licensed vaccine against TB, the Mycobacterium bovis bacilli Calmette–Guérin (BCG) vaccine, it has several limitations, namely its high variability of efficacy in the population and low protection against pulmonary tuberculosis. New vaccines for TB are needed. The World Health Organization (WHO) considers the development and implementation of new TB vaccines to be a priority. Subunit vaccines are promising candidates since they can overcome safety concerns and optimize antigen targeting. Nevertheless, these vaccines need adjuvants in their formulation in order to increase immunogenicity, decrease the needed antigen dose, ensure a targeted delivery and optimize the antigens delivery and interaction with the immune cells. This review aims to focus on adjuvants being used in new formulations of TB vaccines, namely candidates already in clinical trials and others in preclinical development. Although no correlates of protection are defined, most research lines in the field of TB vaccination focus on T-helper 1 (Th1) type of response, namely polyfunctional CD4+ cells expressing simultaneously IFN-γ, TNF-α, and IL-2 cytokines, and also Th17 responses. Accordingly, most of the adjuvants reviewed here are able to promote such responses.
Download full-text PDF here.
2020
Schematic of therapeutic molecules targeting siglec receptors.
Current Status on Therapeutic Molecules Targeting Siglec Receptors María Pia Lenza, Unai Atxabal, Iker Oyenarte, Jesús Jiménez-Barbero and June Ereño-Orbea. Cells 2020, 9(12), 2691; https://doi.org/10.3390/cells9122691.
The sialic acid-binding immunoglobulin-type of lectins (Siglecs) are receptors that recognize sialic acid-containing glycans. In the majority of the cases, Siglecs are expressed on immune cells and play a critical role in regulating immune cell signaling. Over the years, it has been shown that the sialic acid-Siglec axis participates in immunological homeostasis, and that any imbalance can trigger different pathologies, such as autoimmune diseases or cancer. For all this, different therapeutics have been developed that bind to Siglecs, either based on antibodies or being smaller molecules. In this review, we briefly introduce the Siglec family and we compile a description of glycan-based molecules and antibody-based therapies (including CAR-T and bispecific antibodies) that have been designed to therapeutically targeting Siglecs.
Download full-text PDF here.
The immunological lifecycle of Mycobacterium tuberculosis and the progression of the disease.
Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis Chiara Bellini and Kata Horváti. Cells 2020, 9(12), 2673; https://doi.org/10.3390/cells9122673.
The World Health Organization (WHO) herald of the “End TB Strategy” has defined goals and targets for tuberculosis prevention, care, and control to end the global tuberculosis endemic. The emergence of drug resistance and the relative dreadful consequences in treatment outcome has led to increased awareness on immunization against Mycobacterium tuberculosis (Mtb). However, the proven limited efficacy of Bacillus Calmette-Guérin (BCG), the only licensed vaccine against Mtb, has highlighted the need for alternative vaccines. In this review, we seek to give an overview of Mtb infection and failure of BCG to control it. Afterward, we focus on the protein- and peptide-based subunit vaccine subtype, examining the advantages and drawbacks of using this design approach. Finally, we explore the features of subunit vaccine candidates currently in pre-clinical and clinical evaluation, including the antigen repertoire, the exploited adjuvanted delivery systems, as well as the spawned immune response.
Download full-text PDF here.
The main P. aeruginosa virulence factors involved in pathogenesis during pulmonary infections. The components highlighted with syringes have already been evaluated as vaccine antigens.
Understanding Pseudomonas aeruginosa–Host Interactions: The Ongoing Quest for an Efficacious Vaccine Maite Sainz-Mejías, Irene Jurado-Martín and Siobhán McClean. Cells 2020, 9(12), 2617; https://doi.org/10.3390/cells9122617.
Pseudomonas aeruginosa is a leading cause of chronic respiratory infections in people with cystic fibrosis (CF), bronchiectasis or chronic obstructive pulmonary disease (COPD), and acute infections in immunocompromised individuals. The adaptability of this opportunistic pathogen has hampered the development of antimicrobial therapies, and consequently, it remains a major threat to public health. Due to its antimicrobial resistance, vaccines represent an alternative strategy to tackle the pathogen, yet despite over 50 years of research on anti-Pseudomonas vaccines, no vaccine has been licensed. Nevertheless, there have been many advances in this field, including a better understanding of the host immune response and the biology of P. aeruginosa. Multiple antigens and adjuvants have been investigated with varying results. Although the most effective protective response remains to be established, it is clear that a polarised Th2 response is sub-optimal, and a mixed Th1/Th2 or Th1/Th17 response appears beneficial. This comprehensive review collates the current understanding of the complexities of P. aeruginosa-host interactions and its implication in vaccine design, with a view to understanding the current state of Pseudomonal vaccine development and the direction of future efforts. It highlights the importance of the incorporation of appropriate adjuvants to the protective antigen to yield optimal protection.
Download full-text PDF here.
List of inactivated B. pseudomallei vaccine candidates. Other vaccine development strategies include live attenuated vaccines, subunit vaccines, glycoconjugate vaccines, DNA vaccines and viral vector-based vaccines, all discussed in out publication.
Current Advances in Burkholderia Vaccines Development Guanbo Wang, Paulina Zarodkiewicz and Miguel A. Valvano. Cells 2020, 9(12), 2671; https://doi.org/10.3390/cells9122671.
The genus Burkholderia includes a wide range of Gram-negative bacterial species some of which are pathogenic to humans and other vertebrates. The most pathogenic species are Burkholderia mallei, Burkholderia pseudomallei, and the members of the Burkholderia cepacia complex (Bcc). B. mallei and B. pseudomallei, the cause of glanders and melioidosis, respectively, are considered potential bioweapons. The Bcc comprises a subset of Burkholderia species associated with respiratory infections in people with chronic granulomatous disease and cystic fibrosis. Antimicrobial treatment of Burkholderia infections is difficult due to the intrinsic multidrug antibiotic resistance of these bacteria; prophylactic vaccines provide an attractive alternative to counteract these infections. Although commercial vaccines against Burkholderia infections are still unavailable, substantial progress has been made over recent years in the development of vaccines against B. pseudomallei and B. mallei. This review critically discusses the current advances in vaccine development against B. mallei, B. pseudomallei, and the Bcc.
Download full-text PDF here.
Mechanisms of in vitro Bacillus Calmette-Guérin (BCG) training.
Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development Samuel Pasco and Juan Anguita. Cells 2020, 9(9), 2109; https://doi.org/10.3390/cells9092109.
Vaccine design traditionally focuses on inducing adaptive immune responses against a sole target pathogen. Considering that many microbes evade innate immune mechanisms to initiate infection, and in light of the discovery of epigenetically mediated innate immune training, the paradigm of vaccine design has the potential to change. The Bacillus Calmette-Guérin (BCG) vaccine induces some level of protection against Mycobacterium tuberculosis (Mtb) while stimulating trained immunity that correlates with lower mortality and increased protection against unrelated pathogens. This review will explore BCG-induced trained immunity, including the required pathways to establish this phenotype. Additionally, potential methods to improve or expand BCG trained immunity effects through alternative vaccine delivery and formulation methods will be discussed. Finally, advances in new anti-Mtb vaccines, other antimicrobial uses for BCG, and “innate memory-based vaccines” will be examined.
Download full-text PDF here.