The process of designing and testing a vaccine
María García Bengoa
Early Stage Researcher 8, Lionex Diagnostics and Therapeutics
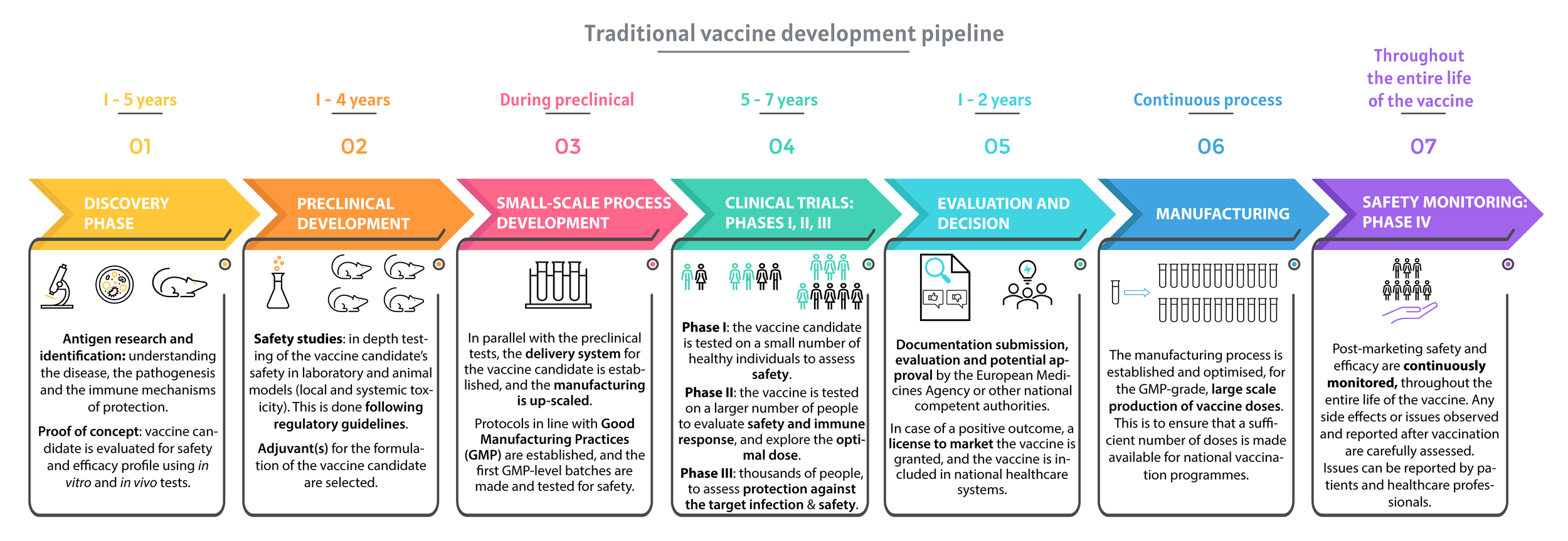
Research and development of a new vaccine, the traditional pipeline.
The figure was redrawn based on infographics from Vaccines Europe, Vaccine Development: From Preclinical Studies to Phase 1/2 Clinical Trials chapter (Springer, 2019, DOI) and SARS-CoV-2 Vaccines in Development (Nature volume 586, pages516–527(2020).
Vaccination represents one of the most effective means of preventing infections in populations. According to the World Health Organization (WHO), immunisation saves three million lives each year, and an additional 1.5 million deaths could be avoided if global vaccination coverage improves. However, every ten seconds someone dies from a vaccine-preventable disease (1).
Designing and testing a vaccine is a slow, complex, and costly process (figure above). Prophylactic vaccines are intended for use in healthy individuals, to prevent diseases, and for this reason they differ from conventional medicines, that are aimed at the treatment of an existing condition (13). Safety is key for vaccines, and for this reason, vaccine clinical trials must demonstrate that the vaccine is both safe and effective in preventing the disease (13).
Developing a human vaccine requires an increasing average investment of approximately US$ 200 to 900 million and a development timeline of 10 to 12 years from the preclinical phase (3, 4). Then, the probability of entering the market is on average as low as 6% (6). Vaccines undergo long and careful review by scientists, doctors, policy makers, regulatory authorities and governments to make sure they are safe and effective before they are recommended and potentially introduced into national immunisation programmes. What is more, vaccine safety is continuously monitored after they are recommended for use, by organisations like the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), and other regulatory agencies and national public health institutes (12).
During all developmental phases of a vaccine, the safety of the new product is a priority, which is why each aspect is strictly regulated (2, 5). The initial research phase (also called ‘discovery phase’, see main figure) usually lasts 1 to 5 years and is followed by preclinical and clinical phases that can last up to 10 years before licensure can be obtained (figure 2). During the research phase, scientists focus on understanding the disease, the pathogenesis and the immune mechanism of protection (13). During this time, candidates are tested for safety in laboratories, in vitro (on cells) and in vivo (on animal models), using different assays.
Before being brought to human studies, vaccine candidates go through a long and rigorous preclinical evaluation, divided between the following phases:
1. Preclinical development
1.1 First step: Defining a Quality Control System for Vaccine Production
The proof of concept refers to how feasible the project is. Once the feasibility is shown, the manufacturer has to establish a quality control approach for the candidate vaccine, in compliance with a set of rules called ‘Good Manufacturing Practices’ (GMP) and in line with the guidelines of the National medicines agency. T hen, the vaccine will be named “released” after validation, and preclinical tests can start (2).
1.2 Second step: Establishing the Safety Profile of the Vaccine Before Testing in Humans
To ensure safety, a panel of toxicity studies must be performed, and animal studies are required for this purpose. Just like every preclinical and clinical testing phase, this step is highly regulated.
Toxicity studies should be designed to mimic the intended use in the clinic, including relevant animal species, dosing schedule and method of vaccine administration. Additional preclinical testing may be required if any signal in preclinical toxicity studies is detected (2).
For ethical reasons, when working with animals, there is a requirement to apply the 3Rs concept of “Replace, Reduce, Refine” in the design of preclinical studies. The 3Rs stand for replacing the use of animals in research, reducing the numbers where the use of animals continues to be required, and refining the care of the animals to keep any pain and suffering to a minimum.
1.3 Third step: Application for approval for Human Trials
Once the small-scale production process is developed and toxicity studies are completed, the sponsor can submit an application for approval to their competent authority and, if approved, clinical trials can start.
2. Clinical evaluation of vaccines
Before human testing can begin, the relevant regulatory authority, such as the EMA in Europe, or the FDA in the US, reviews the preclinical data and the plan for the clinical trial. The sponsor submitting the application describes the manufacturing and testing processes, and details the proposed study, all of which must be approved by the regulatory authority before clinical trials can start (11). The clinical development of a new vaccine candidate follows guidelines that have been issued by the EMA, FDA and the World Health Organization (WHO) (9, 10).
2.1 Phase I (Dose-Escalating Studies)
The objective of Phase I studies is to get as much information as possible on safety and to observe the immune response after the administration of the vaccine formulation. These are dose-escalation studies, which means that the subjects receive single ascending doses of the drug or placebo, the study involving 20 – 80 healthy subjects. The placebo can be an inert substance, such as saline solution, or a well-known vaccine (11). This phase can last up to 1 year. Dose escalation is stopped when maximum tolerability is reached. The best dose is the lowest dose that gives the appropriate immune response with an acceptable safety profile (2). Approximately 70% of drugs move to the next phase (2, 7).
2.2 Phase II
Phase II trials aim to provide further information on safety and immunogenicity. Phase II studies are larger than phase I studies and require hundreds of volunteers with the same characteristics (such as age and sex) as the people for whom the vaccine is intended (8). Phase II studies are divided into two subphases: Phase IIa studies with focus on immune response, dose, and schedule, and phase IIb studies evaluate the efficacy of the vaccine candidate before the initiation of an expensive and complex Phase III trial. Phase IIb trials require long follow-up periods (2). The FDA estimates that only one third of vaccine candidates usually move to the next phase (7).
2.3 Phase III
Successful Phase II vaccine candidates move to Phase III studies, which aim to evaluate a vaccine candidate’s safety and effectiveness in a large cohort (11). This stage requires thousands to tens of thousands of healthy volunteers (11), to identify potentially rare side effects and can last for up to 4 years. Just like Phase II trials, large-scale safety and efficacy studies are usually randomised (subjects are randomly assigned to groups), double-blind (neither the experimenters nor the participants know who is receiving a particular treatment) and placebo-controlled. Efficacy is determined by evaluating the capacity of the vaccine candidate to prevent infection with the pathogen, prevent the disease and induce immune response to the pathogen (production of specific antibodies is such a response). Approximately 25 – 30% of vaccine candidates are approved and move on to phase IV (7). If there are any issues, patients may be exposed to risk or if there is a lack of information, the regulatory authority can place a hold on the trial or ask the developer to pause or stop the trial. Importantly, studies can stop at any stage of the clinical evaluation if potential issues or risks are identified.
3. Safety Monitoring (Phase IV)
If Phase III trials are successful, the vaccine developer can submit a license application to the relevant regulatory authority, who will carefully evaluate all the necessary data and inspect the production facilities of the sponsor (11). Once the drug has been shown to be effective and safe, labelling is the next step, in which the prescribing information is refined, and the developer defines the basis for approval and how to correctly use and administer the vaccine.
Phase 4 trials are carried out once the vaccine candidate has been approved for use. These involve the continuous, long-term monitoring of safety, effectiveness, and production. Once a vaccine is introduced into a national immunisation programme, close monitoring continues to detect any unexpected side effects and further evaluates effectiveness during routine use in an even larger population (8).
Monitoring of the vaccine and production activities must continue for as long as the manufacturer holds a license for the product. Any potential issues or side effects reported to regulatory agencies by healthcare workers (doctors, pharmacists) or individual consumers (patients, parents) are carefully evaluated by an independent advisory group of vaccine safety experts, and immunisation programmes may change as a result.
References:
1. Alliance, G. Advocacy for Immunization: How to generate and maintain support for vaccination programs.
2. Artaud, C., Kara, L., and Launay, O. (2019). Vaccine development: From preclinical studies to phase 1/2 clinical trials. Methods Mol. Biol. 2013, 165–176.
3. Gouglas, D., Thanh Le, T., Henderson, K., Kaloudis, A., Danielsen, T., Hammersland, N.C., Robinson, J.M., Heaton, P.M., and Røttingen, J.A. (2018). Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Heal. 6, e1386–e1396.
4. Maslow, J.N. (2019). Human Vaccines & Immunotherapeutics Challenges and solutions in the development of vaccines against emerging and neglected infectious diseases.
5. Metz, B., Van Dobbelsteen, G. Den, Van Els, C., Van Gun, J. Der, Levels, L., Van Pol, L. Der, Rots, N., and Kersten, G. (2009). Quality-control issues and approaches in vaccine development. Expert Rev. Vaccines 8, 227–238.
6. Pronker, E.S., Weenen, T.C., Commandeur, H., Claassen, E.H.J.H.M., and Osterhaus, A.D.M.E. (2013). Risk in Vaccine Research and Development Quantified. PLoS One 8, 57755.
7. Step 3: Clinical Research | FDA. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process.
8. World Health Organization. How are vaccines developed and tested? https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination?
9. World Health Organization. Guidelines on clinical evaluation of vaccines: regulatory expectations, https://www.who.int/biologicals/publications/clinical_guidelines_ecbs_2001.pdf?ua=1.
10. European Medicines Agency - Committee on Human Medicinal Products (CHMP). Guideline on clinical evaluation of vaccines, https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-evaluation-vaccines-revision-1_en.pdf.
11. The History of Vaccines. Vaccine Development, Testing, and Regulation, https://www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation.
12. Vaccines.gov. VaccinesSafety, https://www.vaccines.gov/basics/safety.
13. Vaccines Europe. How are vaccines developed, https://www.vaccineseurope.eu/about-vaccines/how-are-vaccines-developed.
-
RT @mcclean_siobhan: Our fantastic @Bactivax meeting is all over. Great science, great chats and plenty of fun. Thanks to Rita Berisio (… https://t.co/sC7ddNvinE
-
RT @mcclean_siobhan: We had a really informative session this morning on D3 of our @BactiVax summer school: Science Communication & over… https://t.co/sj3zYNHs0h
-
RT @mcclean_siobhan: Amazing talk from Mariagrazia Pizza from @GSK who shared her extensive experience in working on Bacterial #Vaccines… https://t.co/Nm4U46hxJd
-
RT @mcclean_siobhan: Amazing day and it’s been wonderful to see the research projects of each #ESRs develop and grow. 🎉🎉🎉 @REA_research
-
RT @mcclean_siobhan: @LorenzoBossi5 was our last speaker of the day. Based at @immunxperts, he presented his work on the human immune re… https://t.co/THA1OkaxT3