An arsenal of bacterial defences - the bad, the worse and the silver lining
FRANZISKA PIEPER
EARLY STAGE RESEARCHER 9, IMPERIAL COLLEGE LONDON
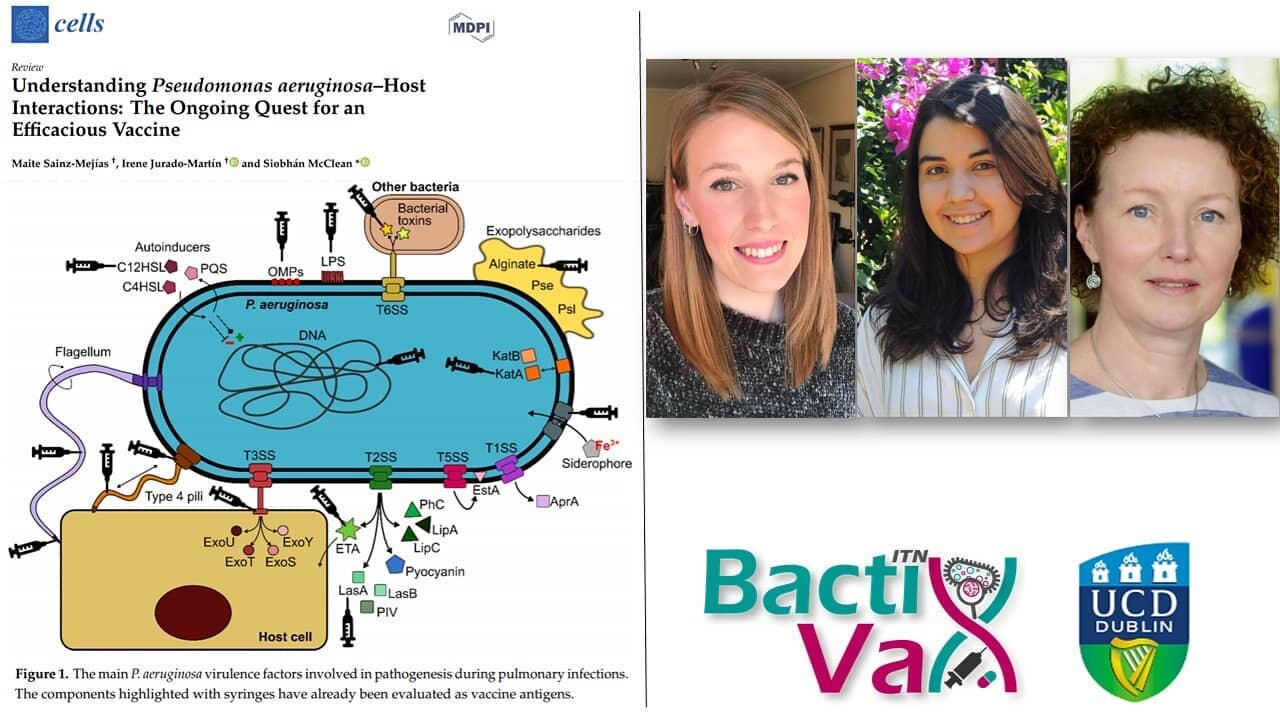
Have you ever had an infection in your ear, lung or anywhere else, that seemed so easy to treat but the doctors around you couldn’t help anymore? Have you ever had to take a cocktail of different antibiotics without knowing whether it will help or make things worse? If not, you belong to the lucky ones, because for thousands of people that is the reality. Our society makes us believe that with all our technological and medical advancements we are invincible, we can conquer all. But that is not the whole truth. We are not the only ones evolving and building defences. Bacteria evolve alongside us and every time you encounter antibiotics, your microbiota does so as well, so do all the microorganisms that are downstream. Bacteria evolve at a steady state, finding ways around the very limited variety of antibiotics we throw at them. Those “ways around” are called virulence adaptations and most of the bacteria we know in a clinical setting have them. Especially, the so often talked about ESKAPE pathogens (Entercoccus faecium, Stahylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp.) acquired ways to escape our efforts of eradicating them.
A virulence adaptation is usually a trait that pathogens use or can acquire to enhance their chances to survive in the host, in you. Most of the time our immune system is strong enough to detect those often-ubiquitous pathogens and eradicate them. But if the immune system is for any reason compromised or overwhelmed, those pathogens can start their attack into a chronic infection. One of the most important traits that can make a difference between clearance of an infection or persistence is a lifestyle change from a mobile lifestyle into a sessile one. This sessile lifestyle is often accompanied by the excretion of a matrix of different carbohydrates and molecules, which leads to the formation of a biofilm. Biofilms are one of the most dangerous adaptations an organism can acquire, because it not only provides the pathogen with an environment to thrive in, but it also decreases the chances of any antibiotics or immune cells to get to the pathogens and kill them.
Other virulence factors are secreted molecules. These molecules can have a direct effect on the host, e.g., proteases and elastases that can break down physical barriers or toxins that can directly poison the host.
Another very important virulence trait are quorum sensing molecules. Those molecules allow bacteria to communicate among each other and sense the density of a given population. Upon sensing these molecules at a certain concentration, the bacteria can upregulate independent virulence factors such as the production of toxins or biofilm. Additionally, many bacteria do not only secrete their preciously manufactured molecules and toxins into the environment and hope for them to arrive at the intended location, but they have special secretion systems to make sure those molecules end up where they are supposed to.
Often bacteria can also develop ways to directly become immune to the antibiotics we use. This can either be through strengthening their cell membranes to become less penetrable, through changing the metabolic pathways that get attacked by the antibiotics, so that the drug can’t act anymore or through efflux pumps that simply transport the antibiotics out of the cell before they can induce damage. These are just some of the most common virulence adaptations, but there are many more and many very specific ones.
I know it is a scary thought, that someday we might be defenceless against this vast variety of pathogens. But that’s exactly why it is so important to understand all the ways pathogens can counteract our defences. We can use this knowledge to find new antibiotics (a quite difficult process), or as we try here at BactiVax, find vaccines to not let it come as far an established infection. Other ways to use virulence adaptations against the bacterium itself are new treatment approaches, like interrupting the communication between bacteria by using specific inhibitors; but this approach is likely still years away due to unknown effects on the dynamic of mixed communities and a lack of actual efficacy testing in humans (Defoirdt, 2018).
Usage of virulence adaptations in vaccine formulations on the other hand, is an approach that has been successfully tried and is used around the globe.
An example of a very successful vaccine formulation incorporating virulence adaptations is the tetanus vaccination. Used against Clostridium tetani, it consists of the chemically inactivated tetanus toxin and is widely used; chances are, you have encountered this vaccine yourself. Vaccinations for diphtheria and pertussis are similarly successful and based on the same principle.
Similarly, there are many vaccines in the making, that use virulence adaptations as antigens:
For Pseudomonas aeruginosa some of their flagella proteins (flagella are the structure with which these bacteria can move) have been identified as potential antigens for vaccine formulation and one of the formulations even reached phase 3 trials (Doering et al., 2007). Andersson et al., (2017) successfully tested a secretion system mutant of Yersinia pestis, the causative agent of the plaque, in a mouse model. The live-attenuated vaccine did not only show 55-100 % protection against the fully equipped strain upon re-challenge, but also presents differences in how macrophages deal with the pathogen.
With researchers worldwide working on solutions to counteract bacteria and their virulence adaptations it is only a matter of time until the next breakthrough.
References:
Andersson, J.A.; Sha, J.; Erova, T.E.; Fitts, E.C.; Ponnusamy, D.; Kozlova, E.V.; Kirtley, M.L.; Chopra, A.K.; Indetification of New Virulence Factors and Vaccine Candidates for Yersinia pestis. Frontiers in Cellular and Infection Microbiology, October 2017, 7, 448
Defoirdt, T.; Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends in Microbiology, April 2018, 26, 313-328
Döring, G.; Meisner, C.; Stern, M.; Flagella Vaccine Trial Study Group. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2007, 104, 11020–11025.
Recent Tweets from BactiVax
-
RT @mcclean_siobhan: Our fantastic @Bactivax meeting is all over. Great science, great chats and plenty of fun. Thanks to Rita Berisio (… https://t.co/sC7ddNvinE
-
RT @mcclean_siobhan: We had a really informative session this morning on D3 of our @BactiVax summer school: Science Communication & over… https://t.co/sj3zYNHs0h
-
RT @mcclean_siobhan: Amazing talk from Mariagrazia Pizza from @GSK who shared her extensive experience in working on Bacterial #Vaccines… https://t.co/Nm4U46hxJd
-
RT @mcclean_siobhan: Amazing day and it’s been wonderful to see the research projects of each #ESRs develop and grow. 🎉🎉🎉 @REA_research
-
RT @mcclean_siobhan: @LorenzoBossi5 was our last speaker of the day. Based at @immunxperts, he presented his work on the human immune re… https://t.co/THA1OkaxT3
-
RT @mcclean_siobhan: How lucky we are, dinner at Punto Chiarito with @BactiVax #ESRs and #PIs https://t.co/SBgZc8R4Xi